Glioblastoma is an aggressive form of cancer that attacks the brain. It is, for now, an extremely difficult disease to overcome — most people live about 15 months after radiation therapy and chemotherapy — but that is very likely about to change, thanks to marijuana. A new treatment incorporating weed’s active compounds has just been shown to be incredibly successful.
On Tuesday, the United Kingdom-based pharmaceutical company GW Pharmaceuticals announced “positive top-line results” from a Phase 2 clinical study in which a combination of tetrahydrocannabinol (THC) and cannabidiol (CBD) were used to treat patients with glioblastoma. THC and CBD are different types of cannabinoids, the psychoactive chemicals found in cannabis.
The clinical study involved 21 patients and culminated in a very encouraging result: People treated with THC and CBD (together with temozolomide, a chemotherapy drug) had an 83 percent one-year survival rate. Those who didn’t get the cannabinoid treatment only had a 53 percent survival rate. The median survival rate for the cannabinoid-treated group was more than 18 months compared to the placebo group’s 12 months.
“Moreover, the cannabinoid medicine was generally well tolerated,” said principal investigator and professor of clinical oncology Susan Short, Ph.D., in a statement. “These promising results are of particular interest as the pharmacology of the THC:CBD product appears to be distinct from existing oncology medications and may offer a unique and possibly synergistic option for future glioma treatment.”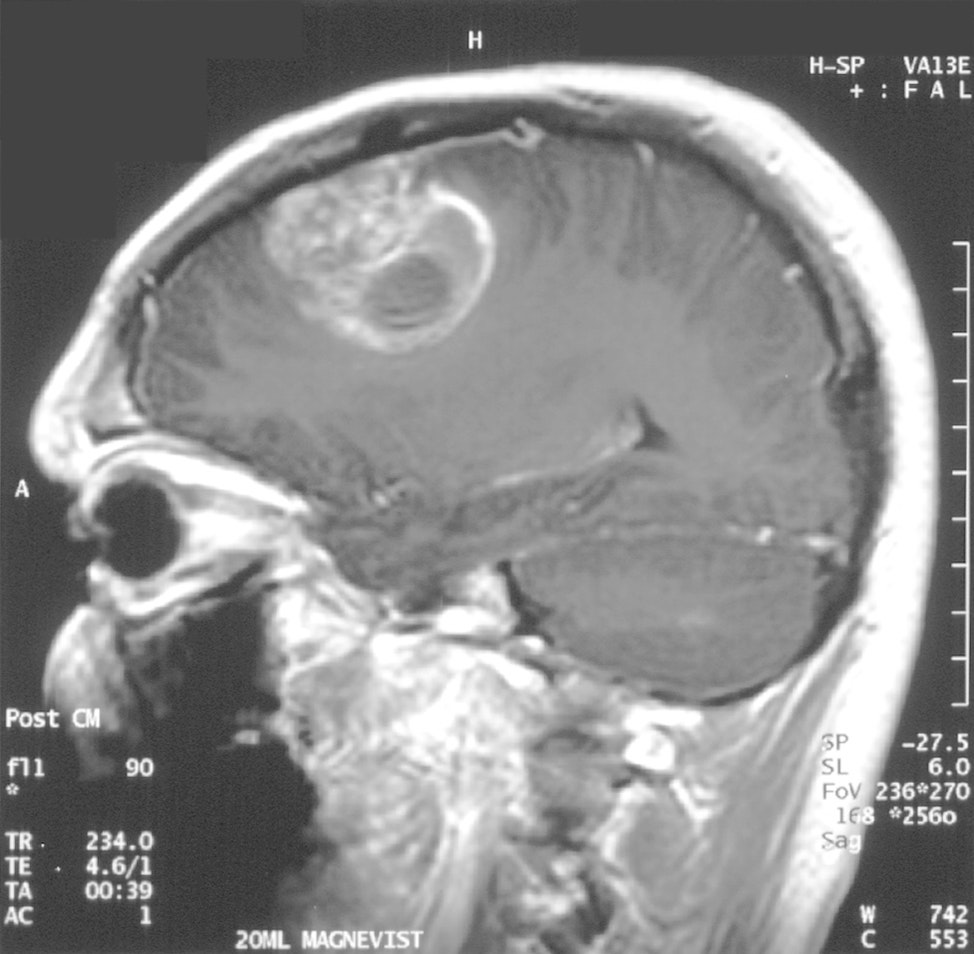
In an announcement, GW’s CEO, Justin Gover, said that this result reinforces the “potential role of cannabinoids in the field of oncology” and will serve as a “catalyst for the acceleration of GW’s oncology research interests over the coming months.” As of now, the company’s cannabinoid treatment has received Orphan Drug Designation from the U.S. Food and Drug Administration, a status assigned to drugs intended to be effective treatments that comes with tax reductions and an exclusive right to develop the cure for a specific condition. In 2015, the U.S. Drug Enforcement Administration eased the regulatory requirements for researchers who want to conduct clinical trials with cannabidiol, like the GW team.
This isn’t the company’s first run at marijuana-based therapy: In 2007, the company began preclinical research involving different cannabinoids and different forms of cancer, which has led to a number of patents and another promising cannabis-based epilepsy drug called Epidiolex.
However, despite all of the promising results tests on cannabis have produced, there’s still a small legislative issue: It’s illegal. As of now, cannabis is not approved by the FDA for use as a cancer treatment, although the American Cancer Society reports that some small studies have demonstrated that cannabinoids like THC and CBD could cause the death of certain types of cancer cells.